Answer:
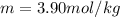
Step-by-step explanation:
Hello there!
In this case, since the molality of a solution is calculated by dividing moles of solute by kilograms of solvent, it turns out firstly necessary for us to calculate the moles of methyl alcohol in 75.0 grams as shown below:
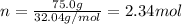
Then, the kilograms of water, 0.600 kg, and finally, the resulting molality:
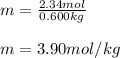
Regards!