Answer:
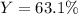
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction:
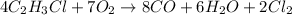
It turns out possible for us to realize about the 7:8 mole ratio of O2 to CO, and therefore, the theoretical yield of the latter is calculated via stoichiometry:
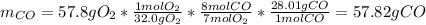
Finally, the percent yield is calculated by dividing the actual yield, 36.5 g by the just computed theoretical one:
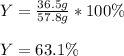
Regards!