Answer:
Because of its weak intermolecular forces.
Step-by-step explanation:
Hello there!
In this case, according to the given description, it turns out possible for us to recall the chemical structures of both ethanol and dimethyl ether as follows:
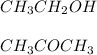
Thus, we can see that ethanol have London dispersion forces (C-C bonds), dipole-dipole forces (C-O bonds) and also hydrogen bonds (O-H bonds) which make ethanol a liquid due to the strong hydrogen bonds. On the other hand, we can see that dimethyl ether has just London and dipole forces, which are by far weaker than hydrogen bonding, that makes it unstable when liquid and therefore it tends to vaporize quite readily.
Regards!