Answer:
See explanation.
Step-by-step explanation:
Hello there!
In this case, for this titration problem, we first need to set up the undergoing chemical equation between barium chloride and hydrochloric acid:
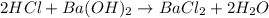
Which occurs in a 2:1 mole ratio of acid to base and thus, we can write the following:
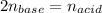
Which can be written in molarities and volumes:
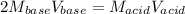
And we solve for the molarity of the acid:
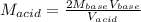
Unfortunately, the molarity of the base was not given:
"The molarity of an aqueous solution of hydrochloric acid, , is determined by titration with a M barium hydroxide, , solution. If 31.2 mL of barium hydroxide are required to neutralize 15.4 mL of the acid, what is the molarity of the hydrochloric acid solution?"
Yet we can assume an arbitrary value, say 1.0 M in order to show you the solution so that you can modify it according to your given value:
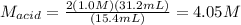
So you just need to modify the (1.0 M) by the actually given molarity of the acid.
Regards!