Answer: The coefficients for the given reaction species are 1, 6, 2, 3.
Step-by-step explanation:
The given reaction equation is as follows.
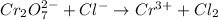
Now, the two half-reactions can be written as follows.
Reduction half-reaction:
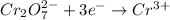
This will be balanced as follows.
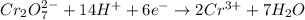 ... (1)
... (1)
Oxidation half-reaction:
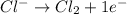
This will be balanced as follows.
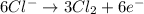 ... (2)
... (2)
Adding both equation (1) and (2) we will get the resulting equation as follows.
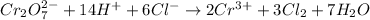
Thus, we can conclude that coefficients for the given reaction species are 1, 6, 2, 3.