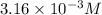Answer: The concentration of
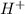 in the solution is
in the solution is
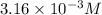
Step-by-step explanation:
pH is defined as the negative logarithm of hydrogen ion concentration present in the solution.
![pH=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/d4u8c7rky5aqengst85apsbxbk128yl4er.png) .....(1)
.....(1)
We are given:
pH of solution = 2.5
Putting values in equation 1, we get:
![2.5=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/xbbd8cspzmag7znu8tdyxp47vjt4gynxb6.png)
![[H^+]=10^(-2.5)](https://img.qammunity.org/2022/formulas/chemistry/college/flrjc3d5izb5mbed4a5q6sz81wdstv159s.png)
![[H^+]=3.16* 10^(-3)M](https://img.qammunity.org/2022/formulas/chemistry/college/m0r9hm65nfjdpkz4mg4d0h644z91crr9f7.png)
Hence, the concentration of
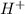 in the solution is
in the solution is