Answer:
23 years
Explanation:
Step 1: Calculate the rate constant (k) for the radioactive decay
A radioactive substance with initial concentration [A]₀ decays to 97% of its initial amount, that is, [A] = 0.97 [A]₀, after t = 1 year. Considering first-order kinetics, we can calculate the rate constant using the following expression.
ln [A]/[A]₀ = - k.t
k = ln [A]/[A]₀ / -t
k = ln 0.97 [A]₀/[A]₀ / -1 year
k = 0.03 year⁻¹
Step 2: Calculate the half-life of the substance
We will use the following expression.
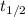 = ln2/ k = ln 2 / 0.03 year⁻¹ = 23 years
= ln2/ k = ln 2 / 0.03 year⁻¹ = 23 years