Answer:
See solution.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to set up the formula for the calculation of the by-mass percentage of the metal:
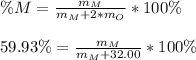
Thus, we solve for the molar mass of the metal to obtain:
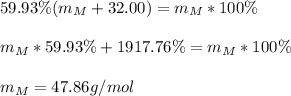
For the subsequent problems, we proceed as follows:
a.
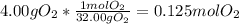
b.
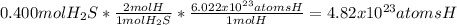
c.
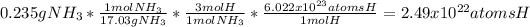
Regards!