The question is incomplete, the complete question is:
When 177. g of alanine
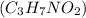 are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is
are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is
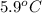 lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is
lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is
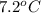 lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.
lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.
Answer: The van't Hoff factor for potassium bromide in X is 1.63
Step-by-step explanation:
Depression in the freezing point is defined as the difference between the freezing point of the pure solvent and the freezing point of the solution.
The expression for the calculation of depression in freezing point is:
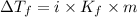
OR
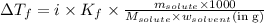 ......(1)
......(1)
- When alanine is dissolved in mystery liquid X:
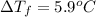
i = Vant Hoff factor = 1 (for non-electrolytes)
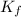 = freezing point depression constant
= freezing point depression constant
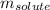 = Given mass of solute (alanine) = 177. g
= Given mass of solute (alanine) = 177. g
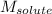 = Molar mass of solute (alanine) = 89 g/mol
= Molar mass of solute (alanine) = 89 g/mol
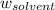 = Mass of solvent = 800.0 g
= Mass of solvent = 800.0 g
Putting values in equation 1, we get:
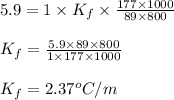
- When KBr is dissolved in mystery liquid X:
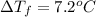
i = Vant Hoff factor = ?
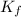 = freezing point depression constant =
= freezing point depression constant =
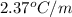
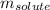 = Given mass of solute (KBr) = 177. g
= Given mass of solute (KBr) = 177. g
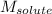 = Molar mass of solute (KBr) = 119 g/mol
= Molar mass of solute (KBr) = 119 g/mol
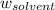 = Mass of solvent = 800.0 g
= Mass of solvent = 800.0 g
Putting values in equation 1, we get:
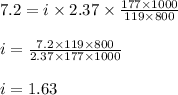
Hence, the van't Hoff factor for potassium bromide in X is 1.63