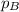The question is incomplete, the complete question is:
Liquid A is known to have a lower viscosity and lower vapor pressure than Liquid B. Two 10 L glass flasks are evacuated and sealed. 35.0 of Liquid A are injected through the seal into one flask, and 35.0 mL of Liquid B are injected into the other flask. After 30 minutes, the pressures
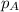 and
and
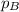 inside the flasks are measured.
inside the flasks are measured.
a.
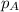 will be greater than
will be greater than
b.
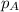 will be less than
will be less than
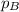
c.
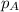 will be equal to
will be equal to
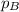
d. It's impossible to predict whether
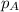 or will be greater without more information.
or will be greater without more information.
Answer: The correct option is b)
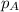 will be less than
will be less than
Step-by-step explanation:
Vapor pressure is defined as the equilibrium pressure which is exerted by the vapor phase to be in thermodynamic equilibrium with its condensed state (liquid phase) in a closed system at a given temperature.
We are given:
Vapor pressure of A < Vapor pressure of B
This means that a lesser number of gaseous molecules will form over its surface and thus, will experience lower pressure.
Thus,
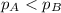
Hence, the correct option is b)
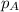 will be less than
will be less than