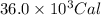Answer: The amount of heat absorbed is
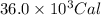
Step-by-step explanation:
Few processes involved are:
(1):
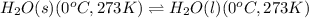
(2):
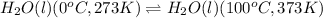
(3):
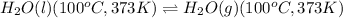
Calculating the heat absorbed for the process having same temperature:
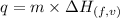 ......(i)
......(i)
where,
q is the amount of heat absorbed, m is the mass of sample and is the enthalpy of fusion or vaporization
Calculating the heat released for the process having different temperature:
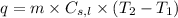 ......(ii)
......(ii)
where,
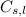 = specific heat of solid or liquid
= specific heat of solid or liquid
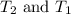 are final and initial temperatures respectively
are final and initial temperatures respectively
We are given:
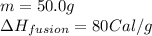
Putting values in equation (i), we get:
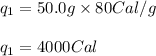
We are given:
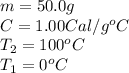
Putting values in equation (i), we get:
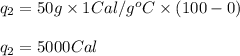
We are given:
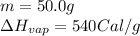
Putting values in equation (i), we get:
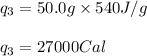
Calculating the total amount of heat released:
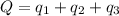
![Q=[(4000)+(5000)+(27000)]Cal=36000Cal=36.0* 10^3Cal](https://img.qammunity.org/2022/formulas/chemistry/high-school/aedcta3rg4zpo8g3vxjwlbiwehepv9mtci.png)
Hence, the amount of heat absorbed is