Answer:
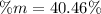
Step-by-step explanation:
Hello there!
in this case, according to the given information, it turns out firstly necessary for us to write up the chemical equation as shown below:
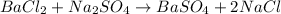
Thus, we calculate the mass of BaCl2 stoichiometrically related to the produced 1.658 g of precipitate in order to discard it from the sample:
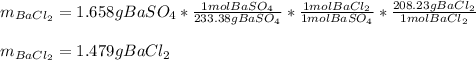
Thus, the mass percentage is calculated as shown below:
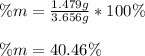
Regards!