Answer:
At temperatures below 924.93K.
Step-by-step explanation:
Hello there!
In this case, according to the thermodynamic definition of the Gibbs free energy of reaction:
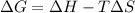
Now, for us to determine the correct temperature range, we must make ΔG=0 and solve for T:
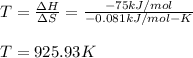
Thus at temperatures below 925.93 K we will have an spontaneous reaction (ΔG<0).
Regards!