Answer: The volume of stock solution needed is 62.5 mL
Step-by-step explanation:
A solution consists of solute and solvent. A solute is defined as the component that is present in a smaller proportion while the solvent is defined as the component that is present in a larger proportion.
To calculate the volume of stock solution needed, the formula used is:
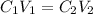 ....(1)
....(1)
where,
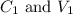 are the concentration and volume of stock solution.
are the concentration and volume of stock solution.
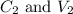 are the concentration and volume of diluted solution.
are the concentration and volume of diluted solution.
We are given:
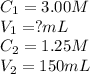
Putting values in equation 1, we get:
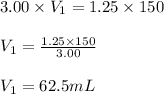
Hence, the volume of stock solution needed is 62.5 mL