Answer: 275.6 L
Explanation: We use Boyle's Law to solve this.
The initial pressure (P1) is 26.5, the initial volume (P1) is 13.0 L
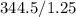 , and the final pressure (P2) is 1.25. Using Boyle's Law, we plug in the values into the equation.
, and the final pressure (P2) is 1.25. Using Boyle's Law, we plug in the values into the equation.
P1V1=P2V2
(26.5 atm)(13.0 L) = (1.25 atm) V2
As a regular equation, we solve for V2.
344.5 atm/L= (1.25 atm) V2
344.5 atm/L / 1.25 atm = V2
275.6 = V2
The atm value cancelled out and we are just left with the L value.