Answer:
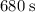 .
.
Step-by-step explanation:
Start by finding the total amount of energy required for melting that much ice.
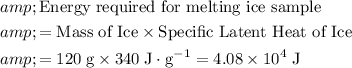 .
.
Hence, the heater would need to supply (at least)
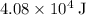 of energy.
of energy.
The power of the heater is
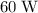 , which is equivalent to
, which is equivalent to
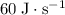 , In other words, the heater is rated to supply
, In other words, the heater is rated to supply
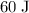 of energy every second.
of energy every second.
Amount of time it takes for the heater to supply
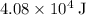 at
at
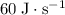 :
:
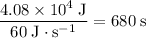 .
.
Hence, it would take
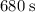 for the heater to melt the ice if the heater is insulated, and all the energy from the heater went to the ice.
for the heater to melt the ice if the heater is insulated, and all the energy from the heater went to the ice.