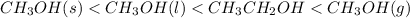Answer: The given compounds are arranged in order of increasing entropy (S) as
Step-by-step explanation:
The degree of randomness present in a substance is called entropy.
This means that more is the number of molecules weakly held together and rapidly moving from one place to another more will be its entropy.
In solids, the molecules are held together strongly with each other. So, they will have least entropy.
In liquids, the molecules are a little loosely held together. So, they have more entropy than a solid substance.
In gases, the molecules are held by weak forces due to which they move rapidly from one place to another. Hence, gases have high entropy as compared to solids and liquids.
Also, more is the molecular mass of a gas less will be its rate of effusion. Hence, less will be its entropy.
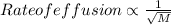
where,
M = molecular mass
Thus, we can conclude that given compounds are arranged in order of increasing entropy (S) as