Answer: The correct option is A).
Step-by-step explanation:
Precipitation reaction is defined as the reaction where a solid precipitate (solid substance) is formed at the end of the reaction. It is insoluble in water.
For the given chemical reactions:
A):
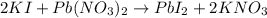
The iodide of lead is generally insoluble in water. Thus, lead iodide is a precipitate.
B):
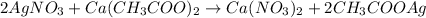
The nitrates and acetates of all metals are soluble in water.
C):
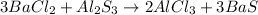
The sulfide of barium is soluble in water.
D):
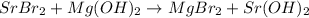
The hydroxide of strontium is soluble in water.
Hence, the correct option is A).