Answer: The %v/v of the given KCl solution is 7.6%.
Step-by-step explanation:
Given: Volume of solute =
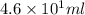
Volume of solution =
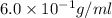
Formula used to calculate %v/v is as follows.
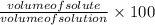
Substitute the values into above formula as follows.
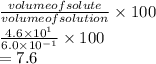
Thus. we can conclude that the %v/v of the given KCl solution is 7.6%.