Answer: The equilibrium constant for the reverse reaction at the same temperature is
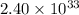 .
.
Step-by-step explanation:
The given reaction equation is as follows.
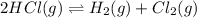 ... (1)
... (1)
It's equilibrium constant (K) value is
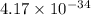 .
.
Also, another reaction equation is as follows.
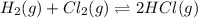
This is the reverse of equation (1). Hence, its equilibrium value is calculated as follows.
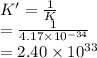
Thus, we can conclude that the equilibrium constant for the reverse reaction at the same temperature is
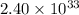 .
.