Answer:
A new fictitious element was discovered.
It is a metal named jolmium, J, and it has three valence electrons.
If it combines with iodine it forms jolmium iodide. Answer the following questions.
a.Is this compound ionic or covalent
b. What is the formula of Jolmium iodide?
c. What is the charge on the metal J in this compound?
d. Explain how you know that is the charge?
Step-by-step explanation:
It is given the element jolmium J is a metal with three valence electrons.
Since metals are highly electropositive, they lose electrons easily and form cations (Ions with a positive charge).
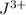 ion will be formed.
ion will be formed.
b) The formula of jolmium iodide is shown below:
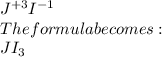
a) The compound is ionic in nature.
Since the compound formed between a metal atom and a nonmetal atom will take place by transfer of electrons.
Hence, it is ionic in nature.
c) The charge on the metal J in this compound is +3.
d) Given J has three valence electrons.
That means it can lose three electrons to form a bond.
So, its valency is three.