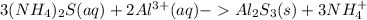Answer:
What happens when one adds (NH4)2S to a solution that contains Al3+?
Step-by-step explanation:
When a solution that contains
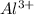 reacts with ammonium sulfide solution, then a precipitate of aluminum sulfide is formed.
reacts with ammonium sulfide solution, then a precipitate of aluminum sulfide is formed.
The chemical equation for the reaction is shown below: