Answer:
H2 + CaCl2 -> 2HCl + Ca
How many grams of HCl are made when 2.93 g of
Ca are made?
Step-by-step explanation:
From the given balanced chemical equation, it is clear that:
2mol. of HCl and 1mol. of Ca are produced.
2mol. of HCl weighs --- 73.0g
1mol. of Ca weighs --- 40.0g
Hence,
73.0g of HCl and 40.0g of Ca are produced.
When 2.93g of Ca is produced then, how many grams of HCl will be produced?
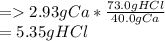
Hence, 5.35g of HCl is formed.