Answer:
See explanation.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary for us to set up the equilibrium reaction by which chromium (II) iodide is ionized as follows:
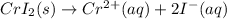
Thus, it is possible to notice that the the moles, and therefore concentrations, of chromium(II) and iodide ions are going to be different, by a factor of 2 due to the mole ratio in the aforementioned chemical equation.
Regards!