Answer:
For A: The wavelength of the light is
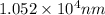
For B: The number of photons per joule is
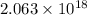
For C: The binding energy of a metal is 1.197 eV.
Step-by-step explanation:
The equation used to calculate the energy of a photon follows:
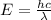 ......(1)
......(1)
where,
E = energy of a photon
h = Planck's constant =
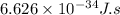
c = speed of light =
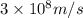
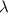 = wavelength
= wavelength
Given values:
E =
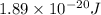
Putting values in equation 1, we get:
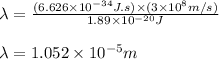
Converting the wavelength into nanometers, the conversion factor used is:
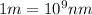
So,
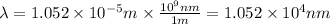
Hence, the wavelength of the light is
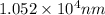
Given values:
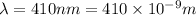
Putting values in equation 1, we get:
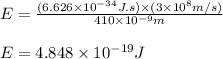
To calculate the number of photons, we use the equation:
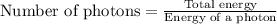
Total energy = 1 J
Energy of a photon =
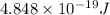
Putting values in the above equation:
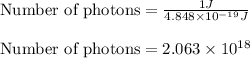
Hence, the number of photons per joule is
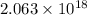
To calculate the binding energy of a metal, we use the equation:
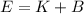 .....(2)
.....(2)
E = Total energy
K = Kinetic energy of a photon
B = Binding energy of metal
Converting the energy from joules to eV, the conversion factor used is:
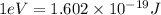
Using the above conversion factor:
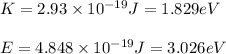
Putting values in equation 2:
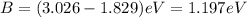
Hence, the binding energy of a metal is 1.197 eV.