Answer:
5 grams of iron react with 5 grams of oxygen to produce iron 3 oxide.
The actual yield of the reaction is--- 5 grams.
What is the percent yield?
Step-by-step explanation:
The balanced chemical equation of the reaction is:
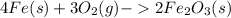
1)Identify the limiting reagent.
2)Using the limiting reagent calculate the amount of theoretical yield formed.
Identification of limiting reagent:
4 mol of Fe reacts with 3mol. of O2
that is:
4mol(55.84g/mol) of Fe reacts with ---- 3mol (32.0g/mol)
=223.36g of Fe reacts with ---- 96g. of O2
then,
5g of Fe requires how many grams of O2?
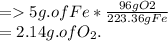
But provided 5g of O2.
So, O2 is present more than required.
Hence, O2 is the excess reagent and the limiting reagent is Fe.
The amount of product formed depends only on the amount of Fe only.
Theoretical yield:
From the balanced chemical equation:
4mol. of Fe forms ----- 2mol. of Fe2O3.
that is
223.36g of Fe forms --- 2(159.68g)of Fe2O3.
=>223.36g of Fe forms --- 319.36g of Fe2O3.
then,
5g of Fe forms ----? grams of Fe2O3
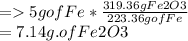
 % error=actual yield/ theoretical yield x 100
% error=actual yield/ theoretical yield x 100
%error=5g./7.14g x100
=>%error=70.0
Hence, the answer is 70.0%.