Answer:
e. 0.175
Step-by-step explanation:
The equilibrium constant of a reaction is the value of its reaction quotient when at equilibrium. The equilibrium concentration of a product or reactant depends on the initial concentrations and equilibrium constant of the reactants and products.
The weak acid approximation depends on the initial concentration and equilibrium constant (
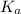 ). The farther the initial concentration and equilibrium constant are from one another, the more likely the approximation would be valid.
). The farther the initial concentration and equilibrium constant are from one another, the more likely the approximation would be valid.