Answer:
Compare the solubility of silver chloride in each of the following aqueous solutions:
a. 0.10 M AgNO3 More soluble than in pure water.
b. 0.10 M NaCI Similar solubility as in pure water
c. 0.10 M KNO3 Less soluble than in pure water.
d. 0.10 M NH4CH3COO
Step-by-step explanation:
This is based on common ion effect.
According to common ion effect, the solubility of a sparingly soluble salt decreases in a solution containing common ion to it.
The solubility of AgCl(s) is shown below:
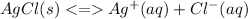
So, when it is placed in:
a. 0.10 M AgNO3
Due to common ion effect Ag+, its solubility is less in this solution than in pure water.
b. 0.10 M NaCI :
Due to common ion effect Cl-, its solubility is less in this solution than in pure water.
c. 0.10 M KNO3 :
In this solution there is no presence of common ion.
So, the solubility of AgCl in this solution is similar to that of pure water.
d. 0.10 M NH4CH3COO:
In this solution, AgCl forms a precipitate.
So, the solubility of AgCl is more in this solution compared to pure water.