Answer: The pH of the resulting solution will be 3.60
Step-by-step explanation:
Molarity is calculated by using the equation:
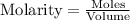 ......(1)
......(1)
We are given:
Molarity of formic acid = 0.100 M
Molarity of potassium formate = 0.100 M
Volume of solution = 420 mL = 0.420 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
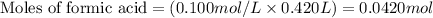
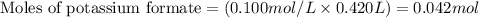
Molarity of KOH = 1.00 M
Volume of solution = 7 mL = 0.007 L
Putting values in equation 1, we get:
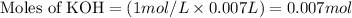
The chemical equation for the reaction of formic acid and KOH follows:
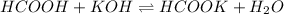
I: 0.042 0.007 0.042
C: -0.007 -0.007 +0.007
E: 0.035 - 0.049
Volume of solution = [420 + 7] = 427 mL = 0.427 L
To calculate the pH of the acidic buffer, the equation for Henderson-Hasselbalch is used:
![pH=pK_a+ \log \frac{\text{[conjugate base]}}{\text{[acid]}}](https://img.qammunity.org/2022/formulas/chemistry/college/mmqbcqjf8qsyvpilhkgby8x0ebxkeuuvve.png) .......(2)
.......(2)
Given values:
![[HCOOK]=(0.049)/(0.427)](https://img.qammunity.org/2022/formulas/chemistry/college/miz6pyuxdgmrrtfg1zlhk2y4017sjrgcsg.png)
![[HCOOH]=(0.035)/(0.427)](https://img.qammunity.org/2022/formulas/chemistry/college/iveve5m87f7je8hoc5xh3zcem9wamgrbii.png)
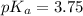
Putting values in equation 2, we get:

Hence, the pH of the resulting solution will be 3.60