Answer:
what is the hybridization of each of the carbon atoms in the compounds
а) CH₃ CH = CH2
b) CH₂=CH2
c) Acetylene. (CH triple bond CH).
Step-by-step explanation:
The hybridization of carbon atom in the organic compounds can be determined by counting the number of surrounding atoms that are in bond with it.
If carbon is directly bonded with two atoms, then it has sp hybridization.
If carbon is directly bonded with three atoms, then it has
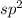 hybridization.
hybridization.
If carbon is directly bonded with four atoms, then it has
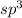 hybridization.
hybridization.
For the given molecules, the hybridization of each carbon atom is shown below: