Answer:
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out necessary for us remember that the first-order kinetics is given by:

Whereas the 27.5% complete means A/Ao=0.275, and thus, we solve for the rate constant as follows:
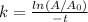
Then, we plug in the variables to obtain:
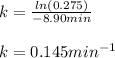
Regards!