Answer:
W = 2 eV
Step-by-step explanation:
Given that,
The wavelength of a photon = 455 nm
The kinetic energy of a photon, K = 0.73 eV
We need to find the work function of the electron. It can be solved using Einstein's equation such that,
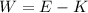
E is the energy of the photon
So,
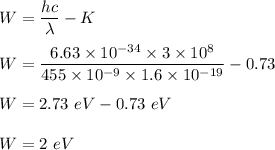
So, the work function of the metal is 2 eV.