Answer:
a. -7.44 °C
Step-by-step explanation:
Hello there!
In this case, since the freezing point depression formula is:
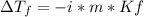
Thus, since the Van't Hoff's factor is 2 for KCl as it ionizes in K⁺ and Cl⁻, the molarity is 2.0 m (2.0mol/1.0kg) and the freezing point depression constant is 1.86 °C/m, we calculate the freezing point depression as follows:
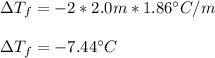
Therefore, the answer is a. -7.44 °C.
Regards!