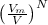Answer:
Step-by-step explanation:
Given :
Number of identical volumes = N
The molecular volume =
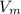
The total volume = V
Temperature = T
Therefore, the number of the ways for locating one molecule within the volume V is :
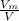
There are N molecules.
So, the total umber of ways of locating N molecules is