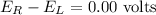Solution :
A cell that is concentrated is constructed by the same half reaction for the anode as well as he cathode.
We know,
In a standard cell,
the reduction half cell reaction is :
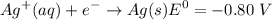
The oxidation half ell reaction :
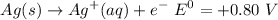
Thus the complete reaction of the cell is :
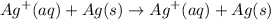
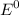 cell =
cell =