Answer:
393 cm
Step-by-step explanation:
Step 1: Given and required data
- Density of liquid methylene bromide (ρ): 2.50 g/mL (2.50 g/cm³)
- Earth's gravity (g): 9.81 m/s²
- Atmospheric pressure (P): 0.950 atm
Step 2: Convert 0.950 atm to Pa (N/m²)
We will use the conversion factor 1 atm = 101325 Pa.
0.950 atm × 101325 Pa/1 atm = 9.63 × 10⁴ Pa
Step 3: Convert 2.50 g/cm³ to kg/m³
We will use the conversion factors:
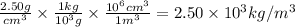
Step 4: Calculate the height (h) of the liquid column
We will use the following expression.
P = ρ × g × h
h = P / ρ × g
h = 9.63 × 10⁴ Pa / (2.50 × 10³ kg/m³) × 9.81 m/s²
h = 3.93 m = 393 cm