Solution :
Comparing the solubility of silver chromate for the solutions :
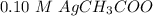 ----- Less soluble than in pure water.
----- Less soluble than in pure water.
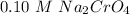 ----- Less soluble than in pure water.
----- Less soluble than in pure water.
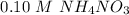 ----- Similar solubility as in the pure water
----- Similar solubility as in the pure water
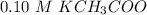 ----- Similar solubility as in the pure water
----- Similar solubility as in the pure water
The silver chromate dissociates to form :
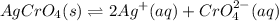
When 0.1 M of
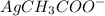 is added, the equilibrium shifts towards the reverse direction due to the common ion effect of
is added, the equilibrium shifts towards the reverse direction due to the common ion effect of
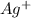 , so the solubility of
, so the solubility of
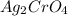 decreases.
decreases.
Both
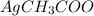 and
and
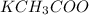 are neutral mediums, so they do not affect the solubility.
are neutral mediums, so they do not affect the solubility.