Answer:
0.565 atm
Step-by-step explanation:
Let's assume we have 100 g of the gas. We'd then have 35.4 g of N₂ and (100 - 35.4) 64.6 g of O₂.
Now we convert those masses into moles, using their respective molar masses:
- 35.4 g N₂ ÷ 28 g/mol = 1.26 mol N₂
- 64.6 g O₂ ÷ 32 g/mol = 2.02 mol O₂
Then we calculate the molar fraction of O₂:
- Molar Fraction O₂ =
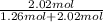 = 0.616
= 0.616
Finally we calculate the partial pressure of O₂:
- Partial P = Total P * Molar Fraction = 0.565 atm