Answer:
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. It can only occur when the element that is doing the replacing is more reactive than the element that is being replaced. Therefore, it is useful to have a list of elements in order of their relative reactivities. The activity series is a list of elements in decreasing the order of reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series.
There are two types of single replacement reactions:
A metal replaces another metal that is in solution:
A + BC → B + AC
Example: Zn + CuC
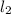 → Cu + ZnC
→ Cu + ZnC
A halogen replaces another halogen that is in solution:
A + BC → C + BA
Example: Br
 + 2KI → I
+ 2KI → I