Answer:
Ksp = 0.0036.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the Ksp of the silver acetate, by knowing that its equilibrium expression is:
![Ksp=[Ag^+][CH_3COO^-]](https://img.qammunity.org/2022/formulas/chemistry/college/h6prvvtkqwaidzancdr4c3qnspbtby0d5e.png)
Next, we calculate molar concentration of the ions in the solution, the same to that of the acetate ions, due to the 1:1 mole ratio, as follows:
![[Ag^+]=[CH_3COO^-]=(1.0g/(166.9122 g/mol))/(0.1000L) =0.0600M](https://img.qammunity.org/2022/formulas/chemistry/college/jubk29635jhc99xp0xutl7hl2xkdxvvof1.png)
Therefore, the Ksp is:
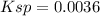
Regards!