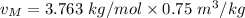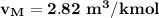Answer:
Step-by-step explanation:
The molar mass of a given substance corresponds and pertains to the unit mole of the mass substance which is stated in g/mol
no of moles of H = mass of H/molar mass of H
= 2 kg/ 2 g/mol
= 2000 g/ 2 g/mol
= 1000 moles
moles of O2 = mass (O2)/ molar mass (O2)
= 2 kg/ 32 g/mol
= 2000 g / 32 g/mol
= 62.5 moles
Total moles present = (1000 + 62.5) moles
= 1062.5 moles
= 1.063 kmol
Total mass = 2kg + 2kg
= 4 kg
no of moles = mass/molar mass
molar mass = mass/ no of moles
molar mass = 4 kg/ 1.063 kmol
molar mass = 3.763 kg/kmol
The specific volume of the final mixture can be determined by the relation:
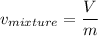
where;
V = 3 m³
m = 4 kg
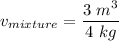
= 0.75 m³/ kg
For the final volume, The molar specific volume is:
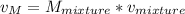
where;
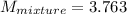
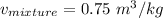
∴