Answer: The correct option is C) decomposition
Step-by-step explanation:
Single displacement reaction is defined as the reaction where a more reactive element displaces a less reactive element from its salt solution.
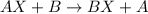
Element B is more reactive than element A
Double displacement reaction is defined as the reaction where an exchange of ions takes place in a solution
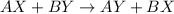
Decomposition reaction is defined as the reaction where a single large chemical species breaks down into two or more smaller chemical species.
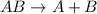
Synthesis reaction is defined as the reaction where two or more smaller chemical species combine together in their elemental state to form a single large chemical species.
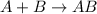
For the given chemical reaction:
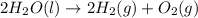
Here, water is breaking down into two smaller species of oxygen and hydrogen gas. Thus, it is a decomposition reaction.
Hence, the correct option is C) decomposition