Answer: Volume of the container is 30.3 L.
Step-by-step explanation:
Given: Mass = 12 g
Temperature =
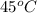 = (45 + 273) K = 318 K
= (45 + 273) K = 318 K
Pressure = 0.544 atm
R = gas constant = 0.0821 L atm/mol K
Moles is the mass of substance divided by its molar mass.
Hence, moles of fluorine (molar mass = 18.99 g/mol) are as follows.
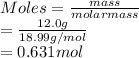
Formula used to calculate the volume of container is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant
T = temperature
Substitute the value into above formula as follows.
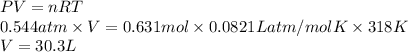
Thus, we can conclude that volume of the container is 30.3 L.