Answer: The value of
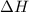 is -163 kJZ
is -163 kJZ
Step-by-step explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
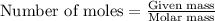 ......(1)
......(1)
We are given:
Given mass of water = 72.0 g
Molar mass of water = 18 g/mol
Putting values in equation 1, we get:
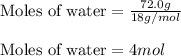
Calculating the heat released for the condensation process:
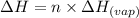 ......(2)
......(2)
where,
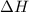 = amount of heat released
= amount of heat released
n = number of moles of water = 4 moles
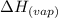 = specific heat of vaporization = -40.7 kJ/mol
= specific heat of vaporization = -40.7 kJ/mol
Negative sign represents the amount of heat released.
Putting values in equation 2:
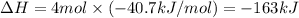
Hence, the value of
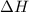 is -163 kJ
is -163 kJ