Answer:
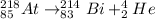
Step-by-step explanation:
Hello there!
In this case, since the radioactive reaction for the alpha emission of astatine-218 to bismith-214 involve the release of a helium atom as shown below:
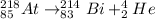
Whereas the atomic number decreases by 2 and the mass number by 4 in agreement to the release of the Helium atom.
Regards!