Answer:
Given the concentration of HCl is 1.5mol/L or 1.5M
The volume of HCl required is --- 80mL
The volume of NaOH is ---- 50mL
What is the concentration of NaOH-- ?
Step-by-step explanation:
The chemical reaction between NaOH and HCl is shown below:
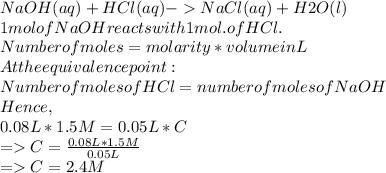
Answer:
The concentration of NaOH required is 2.4M or 2.4mol/L.