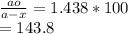Answer:
Given rate constant of the first-order reaction is:
K=7.30x10^-4 s-1
Time t=500s
Determine % H2O2 decomposed in first 500 s in a first-order decomposition reaction of H2O2?
Step-by-step explanation:
The expression for the rate constant of the first-order reaction is:
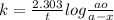
where,
k=rate constant
t=time period
ao=initial amount of the reactant
a-x=amount of a remained after time t.
Substitute the given values in the above formula to get ao/a-x value.
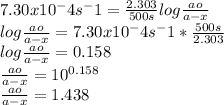
% of H2O2 decomposed is: