Solution :
Given :
Water have quality x = 0.7 (dryness fraction) at around pressure of 200 kPa
The phase diagram is provided below.
a). The phase is a standard mixture.
b). At pressure, p = 200 kPa, T =
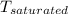
Temperature = 120.21°C
c). Specific volume
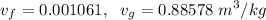
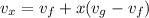
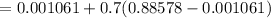
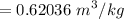
d). Specific energy (
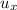 )
)
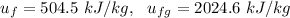
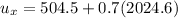
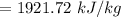
e). Specific enthalpy
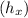
At
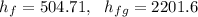
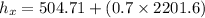
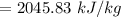
f). Enthalpy at m = 0.5 kg
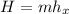
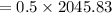
= 1022.91 kJ