Answer:
The amount of NaOH required to prepare a solution of 2.5N NaOH.
The molecular mass of NaOH is 40.0g/mol.
Step-by-step explanation:
Since,
NaOH has only one replaceable -OH group.
So, its acidity is one.
Hence,
The molecular mass of NaOH =its equivalent mass
Normality formula can be written as:
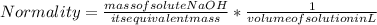
Substitute the given values in this formula to get the mass of NaOH required.
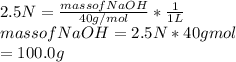
Hence, the mass of NaOH required to prepare 2.5N and 1L. solution is 100g