Answer:
The correct option is (b) "pressure".
Step-by-step explanation:
Gay-Lussac's law states that the pressure of an ideal gas is directly proportional to its temperature at constant mass and volume.
Mathematically, Gay-Lussac's law is as follows :
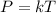
or
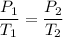
Hence, the correct option is (b) "pressure".